Upsher-Smith Launches Doxazosin Tablets, USP
Upsher-Smith Laboratories, LLC (Upsher-Smith) announced the launch of Doxazosin Tablets, USP, 1 mg, 2 mg, 4 mg and 8 mg. The product was approved by the U.S. Food and Drug Administration (FDA) on April 17, 2018. Upsher-Smith’s Doxazosin Tablets are an AB-rated generic version of the branded product, Cardura (doxazosin mesylate tablets).
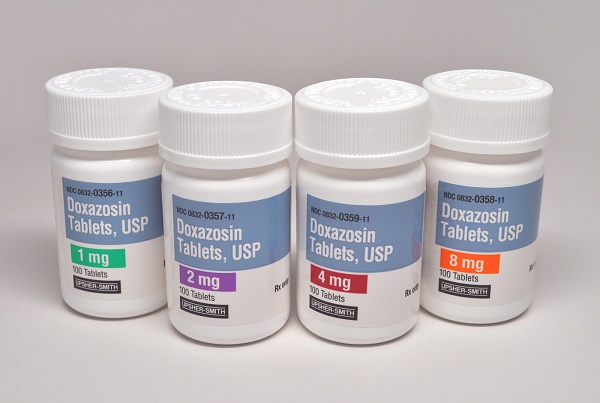
The doxazosin tablet market had U.S. sales of approximately $51.2 million for the 12 months ending January, 2018 according to IQVIA.
Product Information
|
Product |
Active Ingredient |
Strength |
NDC # |
Package Size |
|
Doxazosin Tablets, USP |
Doxazosin mesylate |
1 mg |
0832-0356-11 |
100 ct bottle |
|
Doxazosin Tablets, USP |
Doxazosin mesylate |
2 mg |
0832-0357-11 |
100 ct bottle |
|
Doxazosin Tablets, USP |
Doxazosin mesylate |
4 mg |
0832-0359-11 |
100 ct bottle |
|
Doxazosin Tablets, USP |
Doxazosin mesylate |
8 mg |
0832-0358-11 |
100 ct bottle |

